Detection and quantification of pathological C-terminal TDP-43 fragments in post mortem brain tissue
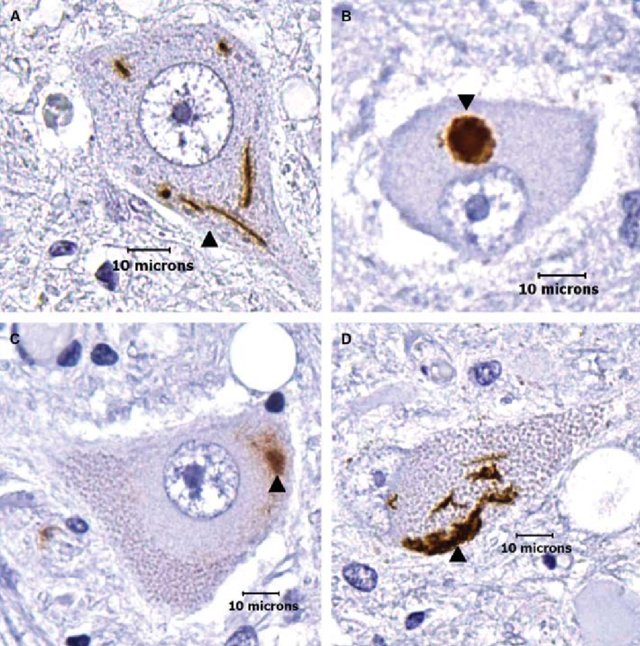 Image credit: Hugo Kim, TDP-43 and ubiquitinated cytoplasmic aggregates in sporadic ALS are low frequency and widely distributed in the lower motor neuron columns independent of disease spread
Image credit: Hugo Kim, TDP-43 and ubiquitinated cytoplasmic aggregates in sporadic ALS are low frequency and widely distributed in the lower motor neuron columns independent of disease spreadBackground:
The pathological hallmark of ALS is the nuclear clearance of TDP-43 in neurons and glia, with cytoplasmic inclusions of post-translationally modified and truncated C-terminal TDP-43 fragments (CTF). The reliable measurement of disease-specific TDP-43 in accessible biofluids would have potential as a biomarker of TDP-43 proteinopathies, but results from antibody-based techniques have been inconsistent to date.
Objectives:
To perform absolute quantification of CTFs using targeted liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Methods:
Biochemical fractionation of post mortem cortical tissue was used to extract soluble and insoluble proteins (urea fraction)(1). Urea fractions were analysed by LC-MS/ MS to detect the peptide coverage of full-length and low molecular weight TDP-43 species. Identified peptide sequences were synthesized with a heavy-label isotope tag for absolute quantification of corresponding endogenous TDP-43 (light) peptides by targeted LC-MS/MS quantitation. Peptide concentrations were determined in ALS-TDP (n=16, with different levels of neuropathological phosphorylated TDP-43 burden), Parkinson’s Disease (PD, n=8), Alzheimer’s Disease (AD, n=8) and healthy control (Ctrl, n=8) urea fractions. The log2 abundance ratio (light peptide/heavy peptide) of healthy controls was compared to all groups using one-way ANOVA Dunnett’s multiple comparison test. CTFs were confirmed by immunoblotting using a C-terminal TDP-43 antibody.
Results:
Peptide sequences covering the N- and the C-terminus of TDP-43, as well as its disease-specific truncation sites, were detected in urea fractions enriched for pathological TDP-43. Compared to healthy controls, quantification of peptides in ALS showed an increase for truncation site-specific peptides (p<0.05). In the sub-group of samples of ALS-TDP (n=8) with more severe phosphorylated TDP-43 pathology, the C-terminal-specific peptides were also increased (p<0.05), as expected. At the same time N-terminal-specific TDP-43 peptides were reduced, possibly reflecting the degradation of the N-terminal cleavage products of TDP-43 (p<0.01). Surprisingly, truncation site specific peptides were increased in the AD urea fractions (p<0.001), but not the C-terminal peptides, suggesting pathological TDP-43 processing in AD cases. Truncation site and C-terminal specific peptides were low abundant in PD and Ctrl urea fractions. In samples where LC-MS/MS quantitation showed increased C-terminal specific peptides, the presence of low molecular TDP-43 fragments was confirmed by immunoblotting (25kDa and 35kDa). In ALS urea fractions the 25kDa band was increased reflecting the abundance of CTFs compared to full-length TDP-43 (p<0.05).
Discussion and conclusions: The detection of specific TDP-43 peptides able to quantify the pathological fragments of TDP-43 in post mortem ALS brain tissue, offers the opportunity to develop an in vivo assay to measure pathological TDP-43. This would have major diagnostic, stratification and pharmacodynamic biomarker potential. The finding of CTF in cases labelled as AD may reflect the recent identification of a sub-group labelled Limbic-predominant Agerelated TDP-43 Encephalopathy.